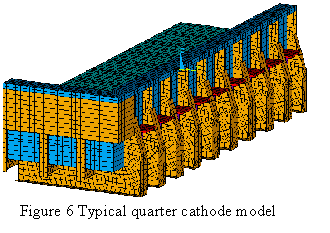
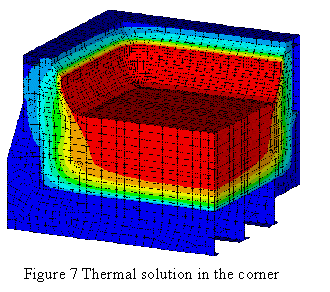
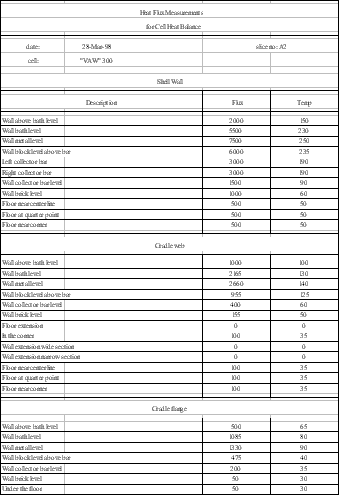
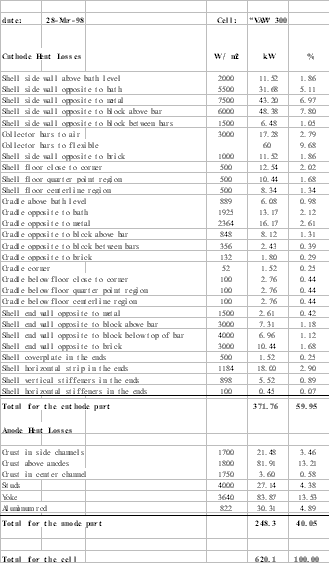
Since Lauralco´s smelter operates the most modern aluminum reduction cell technology available today, the Pechiney´s AP30 cell operating at 300 kA and around 13 kWh/kg, the models must be up to the task of being efficient tools to improve on the forefront of the current best technology available.
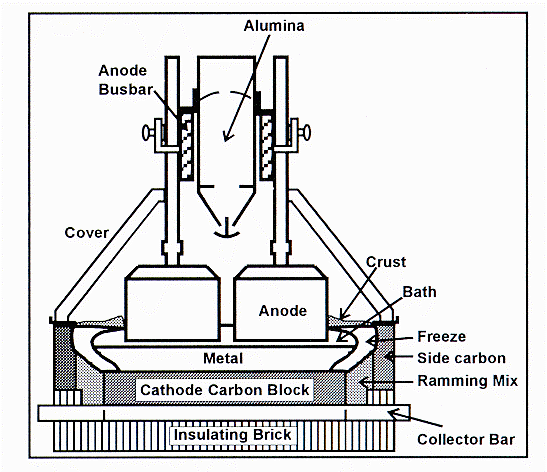
Figure 1: Schematic of an aluminum reduction cell
As we can see in Figure 1, a reduction cell consists of a steel structure called a shell lined with insulating and refractory bricks and finally a thick layer of carbon material. That carbon layer will act as the electric cathode and also as the container for the very corrosive liquid metal and electrolyte. On the top of that, hanging for what is called the cell superstructure, large blocks of carbon partially immerge in the electrolyte layer act as the cell anodes. It is important to know that the carbon anodes are consumed by the electrolysis process and are for that reason manufactured on site and constantly replaced. These anodes, as the cathode cell lining material, must also perform the task of providing thermal insulation since the process must be carried out at a temperature of 960 °C.
Since the anode part of the cell is separated from the cathodic part by the hot liquids zone that does not require to be modeled, it is convenient to build separately models for the anodic and cathodic parts.
Thermo-electric half anode model
The AP30 anode design consists of a prebaked carbon block and a set of three steel studs connected together by a steel yoke. The carbon and the studs are electrically and mechanically joined together by cast iron. The steel yoke is connected to a vertical aluminum rod through a bimetallic joint.
Once the new anode is put in operation in the cell, it is covered by a «crust» of mixed alumina powder and crushed frozen electrolyte to form an insulating layer above the anode. Figure 2 displays the finite element mesh of a typical half anode model.
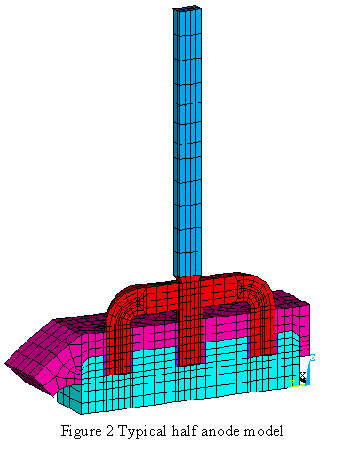
The two key results provided by the model are:
- the total voltage drop across the anode
- the global heat dissipation across the anode panel